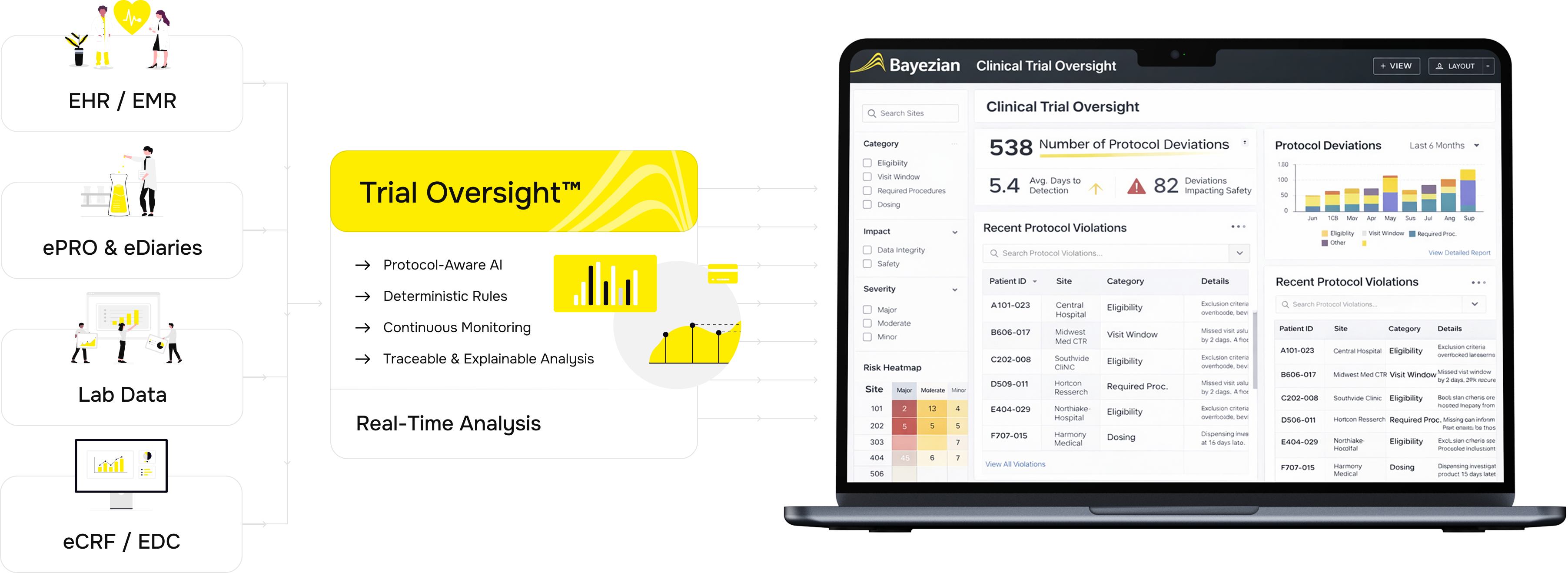
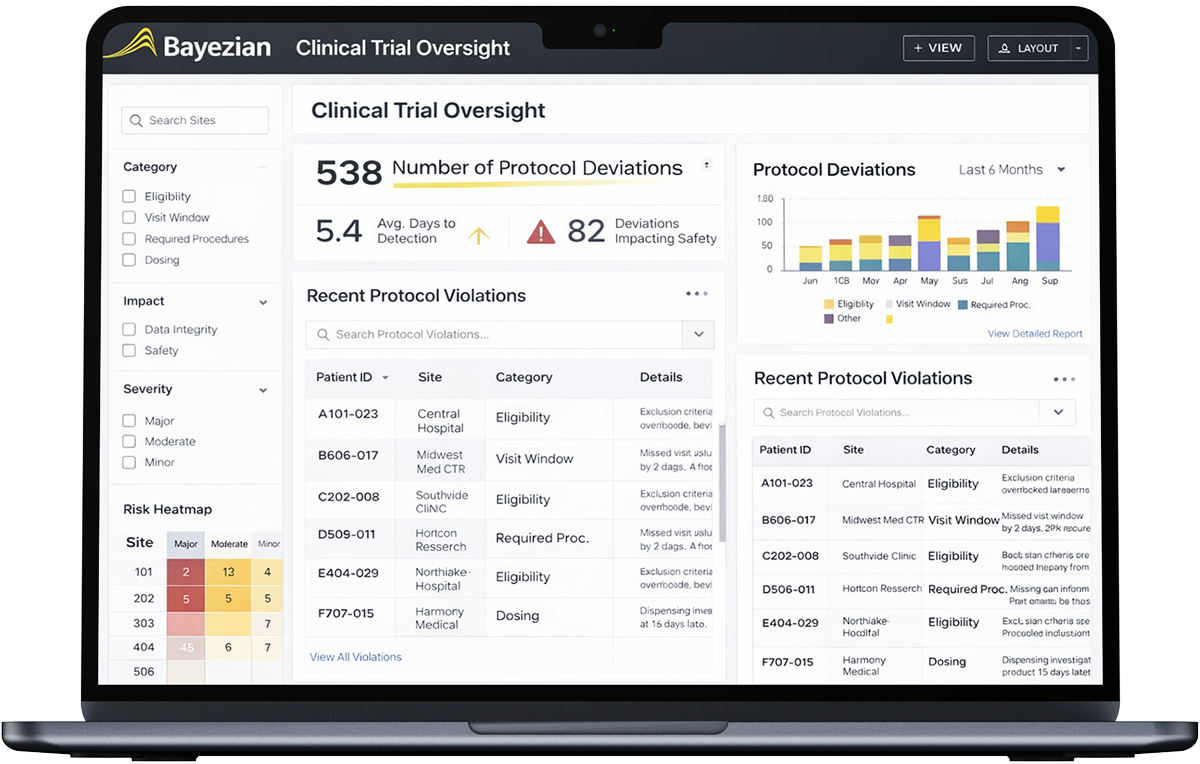
overview
Trial Oversight™ provides continuous, protocol-centred visibility into how clinical trials are executed in practice, enabling earlier identification of risk and stronger control over study quality and compliance.
Traditional Approach
Traditional
Fragmented systems. Retrospective review. Limited early risk visibilty. Clinical trial oversight has not kept pace with increasing protocol complexity and data volume. Most oversight still relies on retrospective review after data entry, limiting the ability to identify and address issues early.
Explore Trial Oversight™